Medical Information

Medical Information Call Centre
Key Features & Benefits
1. Omnichannel Communication:
Supports multiple communication channels, including voice, email, chat, SMS, and social media, ensuring a unified customer experience.
2. Compliance & Security
Fully compliant with HIPAA, GDPR, & DPA, ensuring adherence to key data protection regulations.
Validated for 21 CFR Part 11 and EU Annex 11, guaranteeing compliance with the highest industry standards for regulated environments.
Meets various other security standards, making it suitable for businesses that require stringent compliance.

3. High Availability:
Hosted in a high availability environment with a reliability rate of Four 9's (99.99%) uptime, ensuring minimal disruptions and uninterrupted service for both agents and customers.
4. Disaster Recovery (DR) Capabilities:
Equipped with Disaster Recovery (DR) features to protect business continuity in the event of system failures or other disruptions.
5. Scalability & Flexibility:
As a cloud-based solution, it offers unparalleled scalability, allowing organizations to easily expand operations without compromising performance or security.
6. Real-Time Analytics & Reporting:
Provides real-time performance metrics and reporting tools to help businesses monitor agent efficiency, customer satisfaction, and service levels.
7. Cost Efficiency:
By leveraging the cloud, SentinelRX minimizes infrastructure costs, offering businesses a cost-effective, subscription-based pricing model.
8. Seamless Integration:
Integrates easily with existing business systems, CRMs, and third-party applications, ensuring smooth workflows and data consistency across the organization.
Why Choose
SentinelRX Contact Centre Solution?
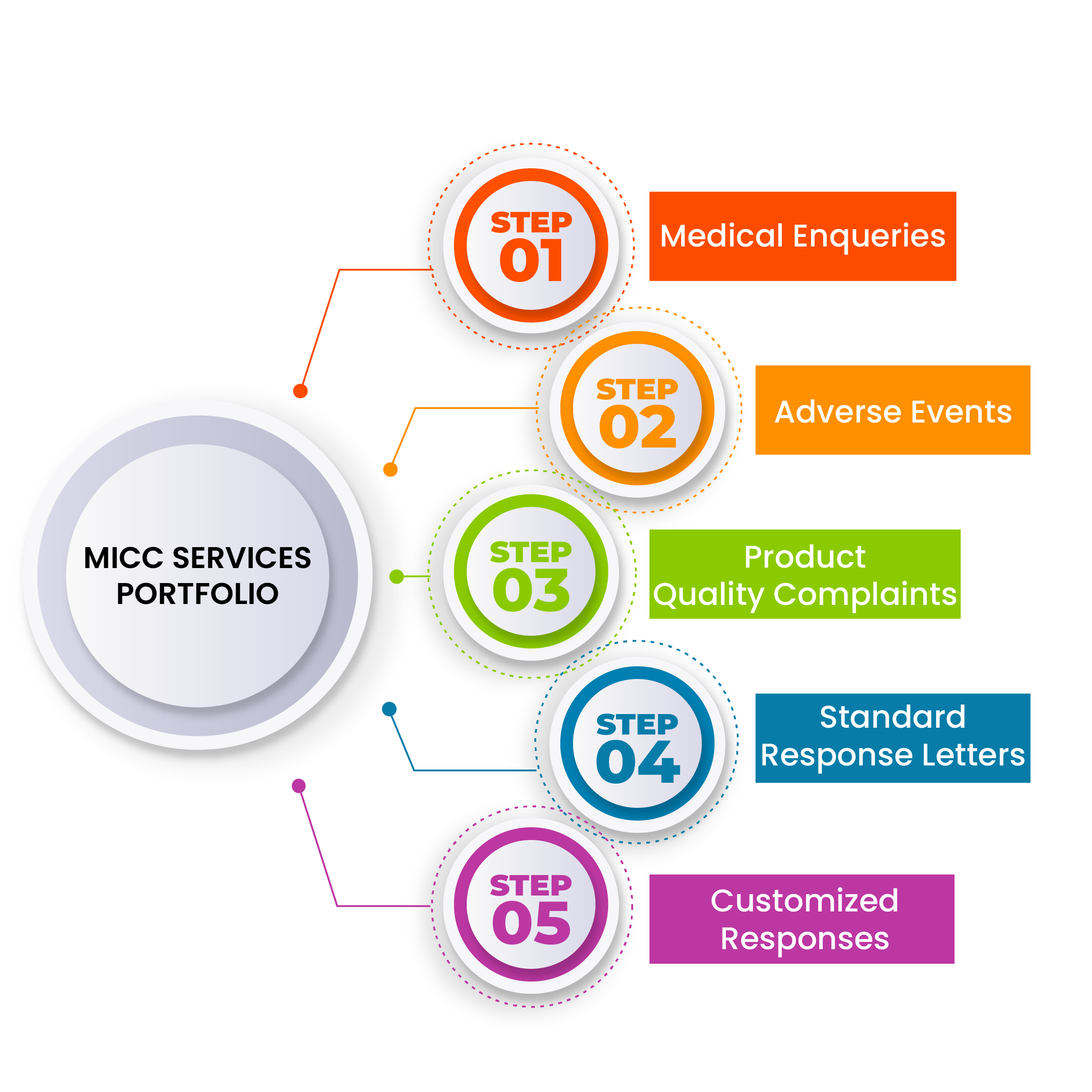
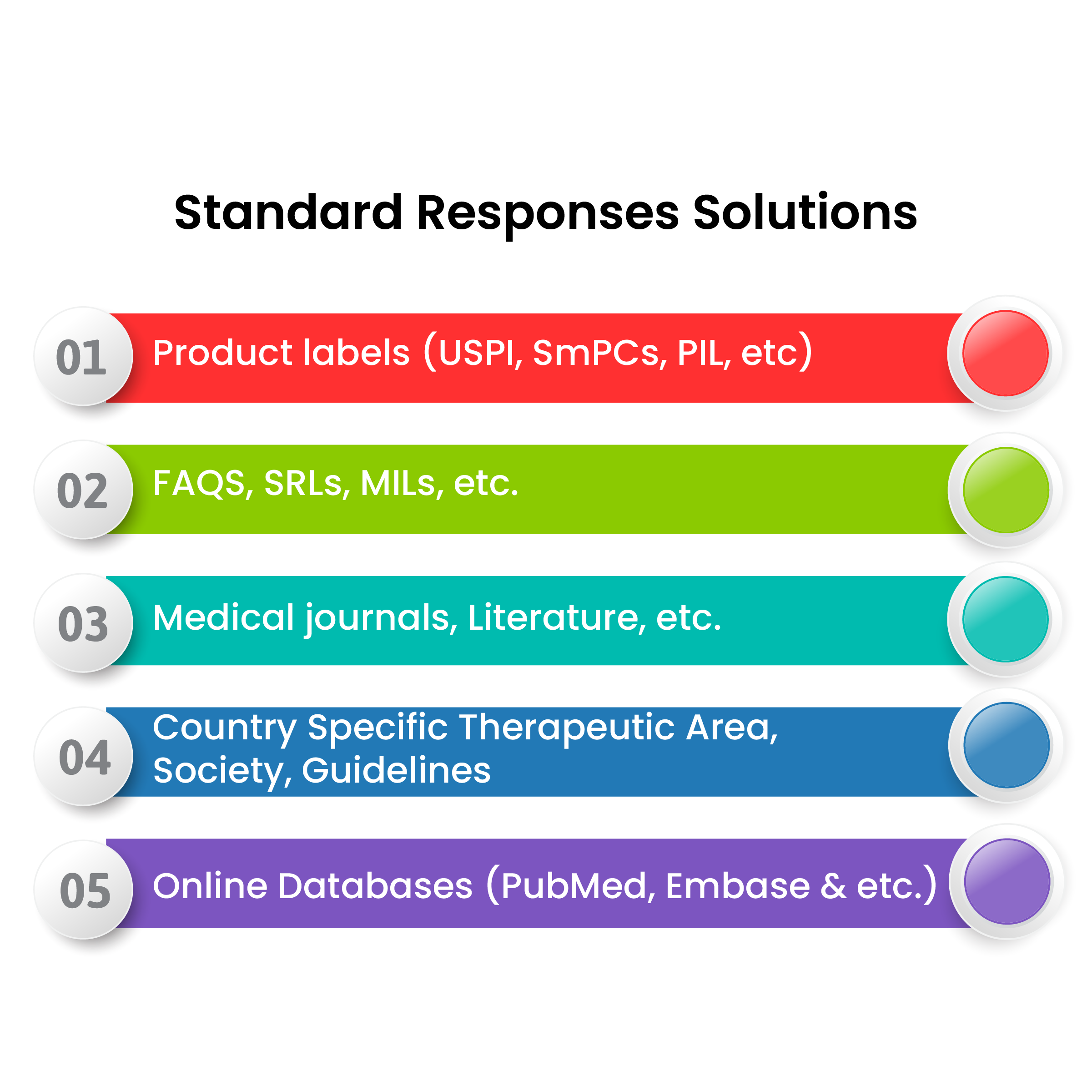
SentinelRX's MICC Advantages
Strategic marketing
Extensive experience in supporting successful Regulatory Inspections
HIPPA Certification
Quality SLAs of 100% for critical fields and >99.6% for non-critical ones, with abandonment rates consistently below 5%.
Global Presence
Integrated Response centre’s presence in US, Philippines, and India with capability to manage overflows/spikes
Medical Information coverage in 50+ countries and 160+ languages.
24x7x365 coverage to address different time zones.
Domain Proficiency
Scalable team models, whether dedicated or shared, are available to meet your needs.
Our offerings include fully customizable modules for Adverse Event (AE), PQC, QA, and Content Management.
Provide specialized support for Risk Evaluation and Mitigation Strategies (REMS) programs, patient support programs, post-authorization registries, and compassionate use programs.
Established processes to efficiently manage requests and inquiries from healthcare providers, patients, consumers, sales representatives, and online
Domain Proficiency
Highly experienced teams of industry/medical professionals
Fully integrated & GAMP-5 validated technology platforms – IRMS, Clinevo MI, ARS MI, etc.
Experience and expertise in different product types including Drugs, Vaccines, Biologics, Biosimilars, Cell and Gene Therapy products/Advanced Therapy Medicinal Products, Medical Devices, and Combination products
